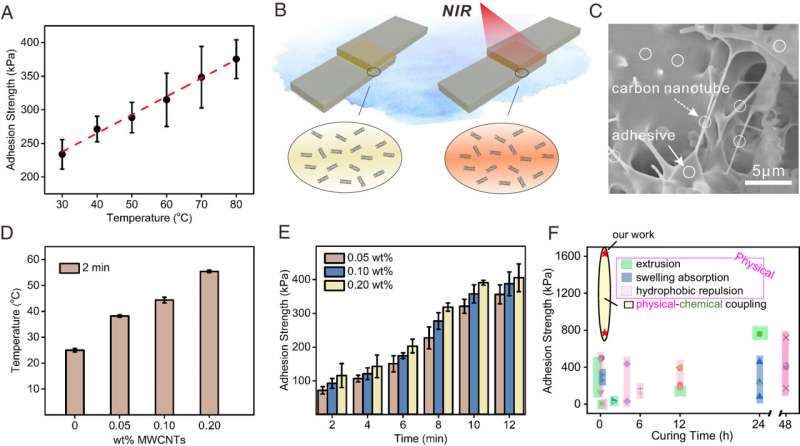
Engineering MWCNTs/SLU-adhesive and realized photo-controlled improvement of adhesion strength by NIR irradiation. (A) Temperature-dependent adhesion strength tests of SLU-adhesive on PI substrate materials for 10 min. (B) Photocontrolled enhancement of the adhesion strength of SLU-adhesive by the photothermal effect of MWCNTs. (C) Scanning electron microscope (SEM) image shows the distribution of doped MWCNTs (0.2 wt%) in MWCNTs/SLU-adhesive. (D) Local temperature change of MWCNTs/SLU-adhesive (0%, 0.05%, 0.1%, 0.2 wt% MWCNTs) after water irradiation for 2 min. (E) With the change of MWCNTs content and irradiation time, the adhesion strength of MWCNTs/SLU-adhesive changed. (F) Comparison of underwater adhesion strength, cure time, and methods to eliminate interfacial water of adhesives that can be used underwater reported in the literature. Credit: Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2301364120
As the interfacial water layer prevents the formation of interfacial contacts and intermolecular interactions between the adhesive and the substrate, in almost all cases of underwater adhesion, water molecules usually act as a destroyer, resulting in poor adhesion. Therefore, the removal of interfacial water from substrate surfaces is essential for the formation of super-strong underwater adhesion.
Despite various physical tests such as absorption, hydrophobic repulsion and extrusion, the complete removal of interfacial water conflict is difficult to achieve due to the inevitable presence of bound water at the interface. Therefore, an unconventional dehydration mechanism with a new material design concept of physical-chemical coupling and a multiscale method is required for deep water removal at the contact interface and self-adaptive cohesion enhancement to create powerful underwater adhesives.
In a study published in PNASZhou Feng’s group from the Lanzhou Institute of Chemical Physics (LICP) of the Chinese Academy of Sciences developed a self-strengthening liquid underwater adhesive (SLU-adhesive) and achieved fast and strong underwater adhesion through deep-water removal of the contact interface and self-adaptive cohesion enhancement.
The researchers used a multiscale dehydration mechanism based on physical-chemical coupling to develop SLU-adhesive. The adhesion mechanism to remove interfacial water includes multiscale dehydration in three steps, that is, the physical replacement of surface water on the substrate with SLU-adhesive on the mm scale due to its excellent wettability, physical removal of water on the μm scale by generating carbon dioxide bubbles from the chemical reaction between the isocyanate groups and water on the surface water level, and simultaneous water level.
This series of adhesion behavior is immediate, spontaneous, and tacitly coordinated throughout the process of liquid adhesive contact, spreading, wetting, and gelation on the surface of the substrate. Strong adhesion of more than 1,600 kPa can be achieved on various materials, including inorganic metals and organic plastic materials, without preloading in various environments such as pure water, a wide range of pH solutions (pH = 3 to 11), and sea water.
Due to their excellent adhesion property and self-adaptive adhesion procedure, SLU-adhesive materials show great potential for a wide range of applications in underwater sand stabilization, underwater repair, and even as a self-reporting adhesive for identifying adhesion failure.
More information:
Chenxi Qin et al, Water-assisted strong adhesion under water by interfacial water removal and adaptive self-gelation, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2301364120
Awarded by the Chinese Academy of Sciences
Citation: Researchers achieve water-assisted strong underwater adhesion (2023, July 25) retrieved 25 July 2023 from https://phys.org/news/2023-07-water-assisted-strong-underwater-adhesion.html
This document is subject to copyright. Except for any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. Content is provided for informational purposes only.
