Adding a flux during the synthesis of oxyhydride is a good strategy to obtain a pure, homogeneous product, revealed scientists from Tokyo Tech. A SrCl2 flux promotes the melting of a part of the reactants and facilitates their diffusion of the reactants, which proved to be the key to the production of extremely pure SrVO2.4H0.6 or Sr3V2O6.2H0.8 perovskite oxyhydride in high-pressure and high-temperature reactions. These compounds have potential as catalysts and as electrode materials for lithium-ion batteries. Credit: Associate Professor Takafumi Yamamoto
Adding a flux during the synthesis of oxyhydride is a good strategy to obtain a pure, homogeneous product, revealed scientists from Tokyo Tech.
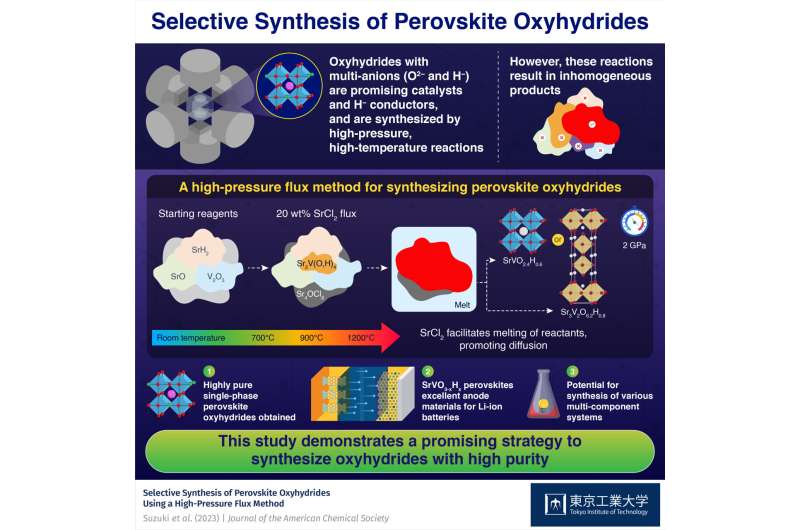
A SrCl2 flux promotes the melting of a portion of the reactants and facilitates their diffusion among the reactants, which has proven to be the key to producing highly pure SrVO2.4H0.6 or Sr3v2or6.2H0.8 perovskite oxyhydride in high pressure and high temperature reactions. These compounds have potential as catalysts and as electrode materials for lithium-ion batteries.
Perovskite oxyhydride with oxide (O2–) and hydride (H–) anions are promising compounds with applications in catalytic systems and batteries. Unfortunately, synthesizing oxyhydride is generally difficult, mainly due to the highly reactive nature of H.– here.
It is known that high pressure and high temperature reactions are effective in synthesizing oxyhydride. For example, Sr2VO4–xHx perovskite can be synthesized directly from oxide and hydride precursors in high-pressure and high-temperature reactions.
An important advantage of these reactions is that H– the content of the final product can be tuned by adjusting the composition and ratio of the precursors. This essentially means that the electronic and magnetic properties of the product can also be customized.
Unlike Sr2VO4–xHxsynthesizes SrVO3–xHx It has proven to be very difficult, because the necessary reactions at high pressure and high temperature lead to the formation of many impurities and inconsistent products, mainly due to the insufficient dispersion of solid components.
In a recent study published in Journal of the American Chemical Society, a research team led by Associate Professor Takafumi Yamamoto from the Institute of Innovative Research at the Tokyo Institute of Technology (Tokyo Tech) found a solution to this problem. They developed a new method to synthesize highly pure SrVO2.4H0.6 and Sr3v2or6.2H0.8, two new perovskite oxyhydrides. This study was conducted as part of a collaborative research project with the National Institutes for Quantum Science and Technology, Japan.
The researchers started with SrO, SrH2and V2or3and SrCl is added2 of these reactants. They observed compositional differences in samples prepared under different conditions using a technique called in-situ synchrotron X-ray diffraction, shedding light on the role of SrCl2 in the reaction. It acts as a flux at a high temperature of 1200 ℃ and a high pressure of 2 GPa, which facilitates the melting and melting of a part of the reactants, thus promoting diffusion.
Because of this, the researchers were able to suppress the development of non-uniform products that are usually seen due to insufficient dispersion, obtaining the purest SrVO2.4H0.6 or Sr3v2or6.2H0.8 perovskite oxyhydride.
In addition, the team investigated the electrochemical properties of the prepared perovskite oxyhydride as an electrode material. “With low working potential, excellent flexibility, and high rate characteristics, SrVO3–xHx may be suitable as a negative electrode for lithium-ion batteries, first for oxyhydride,” said Dr. Yamamoto.
In general, the use of a flux to amplify the desired reaction pathways in high pressure and high temperature reactions can be a powerful strategy to open many new compounds beyond perovskite oxyhydride. Dr. Yamamoto says, “The proposed synthesis method can also be effective in the synthesis of various types of multi-component systems.”
More information:
Selective Synthesis of Perovskite Oxyhydride Using the High-Pressure Flux Method, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c02240
Provided by the Tokyo Institute of Technology
Citation: A high-pressure flux method to synthesize high-purity oxyhydrides (2023, July 25) retrieved on July 25, 2023 from https://phys.org/news/2023-07-high-pressure-flux-method-high-purity-oxyhydrides.html
This document is subject to copyright. Except for any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. Content is provided for informational purposes only.
